Course: “Pre-Clinical and Clinical Safety in Early Development Human Trials”
18-22 March 2024 – 5 days in Paris-Saclay University, France
Registration Deadline 5 March 2024
Registration Deadline 10 February 2023
If you are interested belatedly, please contact us by e-mail
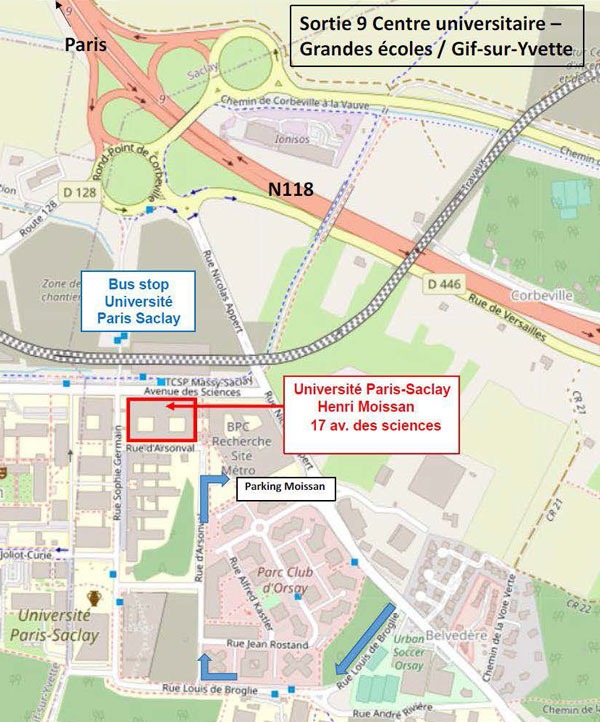
Venue
Faculté de Pharmacie – Université Paris-Saclay
Coeur de pôle – Henri Moissan 2
17 avenue des Sciences
91400 Saclay, France
RER B ‘Robinson’ subway stop
Bus : 194, 294, 379
Highway A86
Access
From Paris CHARLES DE GAULLE AIRPORT or from PARIS center: Take Train RER B direction SAINT-REMY-LES-CHEVREUSES or ORSAY VILLE. STOP at LE GUICHET and then take BUS number 9 direction CAMPUS HEC SACLAY or number 9106 direction CHRIST DE SACLAY. STOP at UNIVERSITE-PARIS SACLAY.
From Paris ORLY AIRPORT: Take ORLYVAL to ANTONY. Take RER B direction SAINT-REMY-LES-CHEVREUSES or ORSAY VILLE. STOP at LE GUICHET and then take BUS number 9 direction CAMPUS HEC SACLAY or number 9106 direction CHRIST DE SACLAY. STOP at UNIVERSITE-PARIS SACLAY.
From Paris center: Take RER B direction SAINT-REMY-LES-CHEVREUSES or ORSAY VILLE. STOP at LE GUICHET and then take BUS number 9 direction CAMPUS HEC SACLAY or number 9106 direction CHRIST DE SACLAY. STOP at UNIVERSITE-PARIS SACLAY.
From Massy TGV train station: take BUS number 9106 direction CHRIST DE SACLAY or LE GUICHET. STOP at UNIVERSITE-PARIS SACLAY.
You can check your itinerary on www.iledefrance-mobilites.fr
Parking Henri Moissan
6 Rue d’Arsonval – Orsay
GPS : 48.711004, 2.175066
Parking in the Henri Moissan building is only possible for those who have specifically asked for a parking place. Because of on-going construction around the building, access to the parking is complicated. Follow the blue arrows on the map.
Coordinator:
Dr Henri Caplain, President AFPT-Club Phase 1 Association française de pharmacologie translationnelle,
hencaplain[@]orange.fr; afpt.cp1[@]gmail.com
Course exclusively in English and in presential (Face2Face) due to its interactive nature. Some parts of the course could be performed on-line.
Minimum number of participants: 10
Course exclusively in English and in presential (Face2Face) due to its interactive nature. Some parts of the course could be performed on-line.
Minimum number of participants: 10
Important note on Covid Regulations in France
Find COVID information regarding traveling to France on Coronavirus – Advice for Foreign Nationals in France – Ministry for Europe and Foreign Affairs (diplomatie.gouv.fr). Please always check the latest updates yourself.
Introduction and Learning Outcomes
This course addresses postgraduates in life sciences interested in early clinical development of medicinal products. The training of several days provides a concise overview on safety in Human Pharmacology / Translational Medicine spanning from non-clinical pharmacology and toxicology over first-in-man to proof-of-concept clinical trials.
Learning Outcomes
On successful completion, students should be able to demonstrate an understanding / knowledge of the following:
- Minimal nonclinical safety package to support the first dose in human (Remember);
- Risk assessment from non-clinical safety package (Apply);
- How to read and understand an Investigator’s Brochure (IB) prior to early clinical trials (Apply);
- Contributing safety findings from early phase trial to the IB (Apply);
- Specific aspects of how-to set-up and conduct safe early phase clinical trials (Apply);
- Selection of appropriate trial population (Understand);
- Assessment, evaluation and reporting of safety data from early clinical trials (Understand);
- Defining pharmacokinetic (PK) endpoints / exposure limit for early phase clinical trials (Apply);
- Safety biomarkers (Understand);
- Development safety update reports (Apply);
- Development of risk management plans (Apply);
- Most important medical emergencies in early clinical trials (Remember);
- Characteristic safety issues involved in the development of biologicals and advanced therapies (Understand).
Minimal pre-training documentation to be covered prior to the training
- M3(R2): Guidance on nonclinical safety studies for the conduct of human clinical trials and marketing authorization for pharmaceuticals (mandatory);
- M7 (R1): Genotoxic impurities (optional);
- S2(R1): Genotoxicity studies (optional);
- S3A, S3B: Toxicokinetics & tissue distribution studies (mandatory);
- S4: Duration of chronic toxicity in animals (rodents & nonrodents toxicity testing) (mandatory);
- S5(R3): Reproductive toxicology (mandatory);
- S6(R1): Preclinical safety evaluation of biotechnology products (optional);
- S7A, S7B: Safety pharmacology studies; QT prolongation (mandatory);
- S8: Immunotoxicology studies for human pharmaceuticals (mandatory):
- S9: Nonclinical evaluation of anticancer pharmaceuticals (optional);
- S10: Photosafety evaluation of pharmaceuticals (optional);
- S11: Nonclinical testing for pediatrics (optional);
- E2F: Development safety update report Step 5 (mandatory);
- E14 (R3): Clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs (mandatory);
- E15: Definitions in pharmacogenetics / pharmacogenomics (optional).
- EMEA/CHMP/28367/07 Rev.1: Guideline on strategies to identify and mitigate risk for first-in-human and early clinical trials with investigational medicine products (mandatory);
- EMEA/CHMP/GTWP/125459/2006: Guideline on the nonclinical studies required before first clinical use of gene therapy medicinal products (optional);
- Guideline on good pharmacovigilance practices (GCP): Module V – Risk management systems (Rev. 2) – 30/03/2017 (mandatory);
- Guideline on good pharmacovigilance practices (GVP): Module VI – Collection, management and submission reports of suspected adverse reactions to medicinal products (Rev. 2) – 02/08/2017 (optional);
- EMEA/CHMP/BMWP/14327/2006 Rev.1: Guideline on immunogenicity assessment of biotechnology-derived therapeutic proteins (mandatory);
- CPMP/EWP/560/Rev.1 Corr.2: Guideline on the investigation of drug interactions (optional);
- EMEA/CHMP/QWP/251344/2006: Guideline on the limits of genotoxic impurities (optional);
- Guideline of CTFG for contraceptive measures (mandatory)
- Guidance for industry: clinical drug interaction studies – Cytochrome P450 Enzyme- and Transporter-Mediated Drug Interactions (January 2020) (optional);
- Guidance for industry: risk evaluation and mitigation strategies: modifications and revisions, rev2 (June 2020) (mandatory);
- Guidance for industry: Safety testing of drug metabolites (22/11/2016) (optional);
- Guidance for industry: estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers (28/07/2005). (mandatory).
in the 2 weeks preceding the start of the course (slide desk).
This AFPT- Le Club Phase 1 course tries to meet the standards for high-quality postgraduate education and training in Medicines Development established by PharmaTrain and the recognition application is proceeding.
Day 1: Monday 18 March 2024
Minimal non-clinical safety package to support the first dose in human
09:00 – 09:15
Coordinator: Henri Caplain, Clinical Pharmacologist, Senior Advisor in Early Clinical Development, Translational Pharmacology, and Drug Safety Risk Management, President AFPT-Le Club Phase 1
09:15 – 11:15
Learning objectives: To provide an understanding/knowledge of general and reproductive toxicology evaluation supporting the first dose in human.
Key concepts: Design of general and reproductive toxicology studies; Dose and species selection; Safety ratio/safety margin; No Observed Effect Level/No Observed Adverse Event Level (NOAEL); Lowest Observed Adverse Effect Level (LOAEL); Maximal Tolerated Dose (MTD); Maximum Feasible Dose (MFD); Limit doses/exposures in repeated -dose toxicity studies; Target organs; Relevance of animal models, including target expression, pharmacodynamics, metabolism and PK aspects, and off-target binding activities and receptor/ligand occupancy and kinetics; Micro-dosing and sub-therapeutic dose concepts and limitations; Juvenile animal testing; Duration of studies to support clinical trials and marketing approval.
Speaker: Philippe Detilleux, Global Head, Preclinical safety, Sanofi R&D
11:15 – 11:30
Coffee Break
11:30 – 12:45
Learning objectives: To provide an understanding/knowledge of pharmacodynamic and safety pharmacology evaluation supporting the first dose in human.
Key concepts: Primary pharmacodynamic studies (in vitro and/or in vivo); Design of safety pharmacology studies; Core battery systems; Assessment of effects on cardiovascular, respiratory and central nervous systems (CNS); Supplemental and follow-up safety pharmacology studies; Secondary organ systems of interest; Use of in silico, animal- and cell-based models of disease mechanisms to study the pharmacology of a new drug.
Speaker: Stephanie Plassman, Specialist in Veterinary Pharmacology and Toxicology, AGAH Regent
12:45 – 14:00
Lunch
14:00 – 16:30
Learning objectives: To provide an understanding/knowledge of nonclinical pharmacology and pharmacokinetic evaluation supporting the first dose in human and PK/PD modelling to bridge nonclinical and safety endpoints.
Key concepts: Assessment of the mode of action/effects of candidate compound on the target; Absorption/distribution/ metabolism and excretion (ADME) assessment; Toxicokinetic evaluation; Half-life, Cmax, systemic exposure (AUC), in vitro metabolic and plasma protein binding for animals and humans, clearance, volume of distribution, intrinsic and extrinsic factors which affect the PK; PK linearity/non-linearity/ Dose-proportionality; Steady-state; Accumulation factors; Metabolites assessment (animals and nonclinical characterization for humans); Pharmacogenetics/polymorphisms/ Pharmacometrics/PK/PD modelling.
Speaker: Jeremy Perrier, PBPK scientist, PhinC Development
16:30 – 16:45
Coffee Break
16:45 – 18:30
Learning objectives: To provide an understanding/knowledge of on- and off-target evaluation before the first use in human.
Key concepts: On- and off-target binding affinities; Receptor/ligand occupancy and kinetics.
Speaker: Friedemann Schmidt, Computational / Systems Toxicologist, Sanofi R&D and Technical University Darmstadt
18:30
Adjourn
Day 2: Tuesday 19 March 2024
Minimal non-clinical safety package to support the first dose in human and principles of risk assessment from non-clinical safety package
09:00 – 10:30
Learning objectives: To provide an understanding/knowledge of evaluation of potential immunotoxicity.
Key concepts: Standard toxicity studies; Study design to assess drug-induced immunotoxicity; Selection of assays; Potential immunotoxicity linked to the pharmacological properties, intended patient population, structural similarity, disposition of the drug.
Speaker: Pr. Marc Pallardy, Dean Faculty of Pharmacy and Director of Interdisciplinary Action “Health and Therapeutic Innovation” Paris-Saclay University
10:30 – 10:45
Coffee Break
10:45 – 12:30
Learning objectives: To provide an understanding/knowledge of nonclinical package require before the first use in human of gene therapy medicinal product.
Key concepts: Pharmacodynamic “proof of concept” in nonclinical model(s); Biodistribution; Studies to establish dose; Toxicity studies for the whole gene therapy medicinal product (virus or other micro-organism or vector particle and/or delivery system + expression vector including cassette + transgene; Integration studies; Germline transmission; Target tissue selectivity; Immunogenicity and immunotoxicity; Delivery devices and excipients; Environmental risk/shedding.
Speaker: Philippe Detilleux, Global Head, Preclinical safety, Sanofi R&D
12:30 – 14:00
Lunch
14:00 – 15:00
Learning objectives: To provide an understanding/knowledge of genotoxicity evaluation supporting the first dose in human and potential genotoxic impurities.
Key concepts: Design of genotoxicity assessment; In vitro and in vivo testing; Genotoxic impurities and threshold of toxicological concern (TTC).
Speaker: Guy Bouvier, Toxicology and Product Safety Director, Pierre-Fabre Laboratories
15:00 – 15:45
Learning objectives: To provide an understanding/knowledge of photosafety testing before the first use in human.
Key concepts: Phototoxicity; Photoallergy; Photogenotoxicity; Photocarcinogenicity; Need for photosafety testing before first in human study; Phototoxicity testing.
Speaker: Béatrice Gauthier, Veterinary Pathologist Expert, Sanofi R&D
15:45 – 16:30
Learning objectives: To provide an understanding/knowledge of nonclinical local tolerance evaluation.
Key concepts: Design and need of local tolerance studies; Sensitizing potential; Oral, ocular, cutaneous tolerance testing; Transdermal systems; parenteral tolerance testing; Rectal and vaginal tolerance testing.
Speaker: Béatrice Gauthier, Veterinary Pathologist Expert, Sanofi R&D
16:30 – 16:45
Coffee Break
16:45 – 18:30
Learning objectives: To provide the principles behind the principal of risk assessment from nonclinical studies.
Key concepts: Importance of toxicokinetic; Risk factors/Safety factor; PK linearity/nonlinearity/dose proportionality/accumulation; Variable bioavailability; Steep dose response curve; Severe toxicities; Non-monitorable toxicities; Reversible/Irreversible toxicities; Toxicities without premonitory signs; Long-lasting binding and effects; Nature of the target and novel therapeutic targets; Differences and similarities between the pharmacology and toxicology of compounds and their metabolites in animals, humans, and cell preparations that provide qualitative and quantitative assessment: genotoxicity, general toxicity, toxicokinetics, pharmacokinetics, drug metabolism, safety pharmacology, immunotoxicity, reproductive toxicity, carcinogenicity; Relevance of nonclinical findings in various organ systems (liver, CNS, endocrine, eye, kidney, reproductive and gastrointestinal tract); Extrapolation of animal findings to human; Differences in nonclinical safety and toxicity packages between small molecules, biological medicines, advanced therapies.
Speaker: Nigel Roome, Toxicology and Toxicologic Pathology Senior Consultant
18:30
Adjourn
Day 3: Wednesday 20 March 2024
Safety in human pharmacology trials
09:00 – 10:30
Learning objectives: To provide an understanding/knowledge of how to perform a safe first-in-human study.
Key concepts: How to read and understand the safety concerns in the first Investigators Brochure (IBs) and its maintenance; General principles of first-in-human studies, including overall design; Estimating the first safe dose in a first-in-human trial, including the concepts of Human Equivalent Dose (HED), Maximum Recommended Starting Dose (MRSD), NOAEL-based approach, Minimal Anticipated Biological Effect (MABEL), Minimum Effective Dose (MED), Pharmacological Active Dose (PAD); Allometric scaling; Sequence and interval between dosing of subjects within the same cohort, concept of sentinel subjects; Safe dose escalation scheme and last dose, including the Anticipated Therapeutic Dose Range (ATD); Minimal clinical evaluations and evaluations depending on the nonclinical findings, including the intensity and duration of monitoring; Safety biomarkers; Stopping rules; How to proceed from single ascending dose to multiple ascending dose – assessment evaluation of SAD safety and PK data, integrated protocols versus consecutive trials (pros, cons and operations); Maximum duration of treatment; Decision making group or safety review committee; Identification of protocol violations and deviations; Safety data: tables and graphs for the evaluation of adverse events, laboratory data and other data related to safety; PD data: tables and graphs for the evaluation of pharmacodynamic.
Speaker: Yves Donazzolo, Principal Investigator Optimed/Eurofins, AFPT-Le Club Phase 1, Past-President EUFEMED
10:30 – 10:45
Coffee Break
10:45 – 11:45
Learning objectives: To provide an understanding/knowledge of how to perform a safe first-in-human study.
Key concepts: How to read and understand the safety concerns in the first Investigators Brochure (IBs) and its maintenance; General principles of first-in-human studies, including overall design; Estimating the first safe dose in a first-in-human trial, including the concepts of Human Equivalent Dose (HED), Maximum Recommended Starting Dose (MRSD), NOAEL-based approach, Minimal Anticipated Biological Effect (MABEL), Minimum Effective Dose (MED), Pharmacological Active Dose (PAD); Allometric scaling; Sequence and interval between dosing of subjects within the same cohort, concept of sentinel subjects; Safe dose escalation scheme and last dose, including the Anticipated Therapeutic Dose Range (ATD); Minimal clinical evaluations and evaluations depending on the nonclinical findings, including the intensity and duration of monitoring; Safety biomarkers; Stopping rules; How to proceed from single ascending dose to multiple ascending dose – assessment evaluation of SAD safety and PK data, integrated protocols versus consecutive trials (pros, cons and operations); Maximum duration of treatment; Decision making group or safety review committee; Identification of protocol violations and deviations; Safety data: tables and graphs for the evaluation of adverse events, laboratory data and other data related to safety; PD data: tables and graphs for the evaluation of pharmacodynamic data.
Speaker: Yves Donazzolo, Principal Investigator Optimed/Eurofins, AFPT-Le Club Phase 1, Past-President EUFEMED
11:45 – 12:30
Learning objectives: To provide the principles of the management of medical emergencies in human pharmacology trials.
Key concepts: Pre-trial interviews and screening procedures; Up-to-date resuscitation procedures and guidelines; Diagnosis and management of anaphylaxis and other severe allergic phenomena, cardiac arrhythmias, respiratory emergencies, syncope, convulsions and other neurotoxicity.
Speaker: Yves Donazzolo, Practitioner Emergency Department, Grenoble University Hospital, AFPT-Le Club Phase 1, Past-President EUFEMED
12:30 – 14:00
Lunch
14:00 – 14:45
Learning objectives: To provide an understanding/knowledge of choice of study population for the first-in-human trial.
Key concepts: Healthy participants versus patients; Inclusion of special population including women, children, elderly, ethnicity, genotype(s), cultural differences, possible interaction with subject’s lifestyle, e.g. smoking, use of alcohol or drugs; Use of other medications with the possibility for adverse reactions and/or difficulties in the interpretation of results; Safety criteria of inclusion and exclusion; How to exclude participants with drug abuse and drug dependence; Protection of research participants; Sponsor and investigator responsibilities in context of trial participants, in particular, to avoid conflicts of interest.
Speaker: Lionel Hovsepian, Clinical Pharmacologist, Early development expert, AFPT-Le Club Phase 1
14:45 – 16:30
Learning objectives: To provide an understanding/knowledge of first-in-human oncology trials.
Key concepts: Trials design, including traditional 3+3 design, Continual Reassessment Method (CRM), Dose Escalation with Overdose Control (EWOC) and other Bayesian approaches; Phase I trials of Agent Combinations; First dose; Dose escalation; Stopping rules; Grading of adverse events including the ‘Common Terminology Criteria for Adverse Events’ (CTCAE) descriptive terminology; Maximal Tolerated Dose (MTD; Dose limiting toxicities (DLTs); Data safety monitoring board (DSMB).
Speaker: Pr. Christophe Massard, Medical Oncologist, Centre Eugene Marquis Rennes
16:30 – 16:45
Coffee Break
16:30 – 18:30
Learning objectives: To provide an understanding about the timing and safety implications of other Phase I trials, how to assess safety findings and individual exposure and an understanding/knowledge of the integrated cardiac safety.
Key concepts: Safe food effect trial; Bioequivalence study; Drug-drug interactions to be performed in Phase I of clinical development; Patients with renal or hepatic impairment; Design and timing of TQT study; Integrated cardiac safety concept.
Speaker: Denis Gossen, Clinical Pharmacologist, AFPT-Le Club Phase 1
18:30
Adjourn
Day 4: Thursday 21 March 2024
Pharmacovigilance in human pharmacology trials
09:00 – 10:30
Learning objectives: To provide an understanding/knowledge of AEs/ADRs evaluation and reporting.
Key concepts: Role of the pharmaceutical professional in drug safety and pharmacovigilance; Methodology for collection in clinical trials, including reporting; Mechanisms of AEs/ADRs/safety risks; Assessment and classification of adverse events (AEs), adverse drug reactions (ADRs), serious adverse events (SAEs), suspected unexpected serious adverse reactions (SUSARs), adverse events of special interests (AESIs);.MedDRA coding and classification; Medical aspects of AEs/ADRs, including principles of event attribution, evidence for association and causality, expectedness and seriousness assessments; The extent of variation in normality.
Speaker: Hervé Bester, VP Global Patient Safety – Rare Diseases Therapeutic Area Head, Ipsen
10:30 – 10:45
Coffee Break
10:45 – 12:30
Learning objectives: To provide an understanding/knowledge of.AEs/ADRs evaluation and reporting.
Key concepts: Role of the pharmaceutical professional in drug safety and pharmacovigilance; Methodology for collection in clinical trials, including reporting; Mechanisms of AEs/ADRs/safety risks; Assessment and classification of adverse events (AEs), adverse drug reactions (ADRs), serious adverse events (SAEs), suspected unexpected serious adverse reactions (SUSARs), adverse events of special interests (AESIs);.Medical aspects of AEs/ADRs, including principles of event attribution, evidence for association and causality, expectedness and seriousness assessments; The extent of variation in normality.
Speaker: Hervé Bester, VP Global Patient Safety – Rare Diseases Therapeutic Area Head, Ipsen
12:30 – 14:00
Lunch
14:00 – 15:45
Learning objectives: To illustrate the potential safety impact of AEs/ADRs.
Key concepts: General tolerability; Tolerance; Liver/renal toxicity, including drug-induced liver injury (DILI); CNS toxicity; Cardiac toxicity, including pro-arrhythmogenic risk; Immune toxicity, including cytokine release syndrome (CRS); Other system or local toxicities of concern; Monitoring of vital signs; What happens in case of pregnancy during a trial; Predisposing factors and the impact of pre-existing disease on the susceptibility for and severity of adverse events.
Speaker: Henri Caplain, Clinical Pharmacologist, Senior Adviser in Early Clinical Development, Translational Pharmacology, and Drug Safety Risk Management, President AFPT-Le Club Phase 1
15:15– 16:30
Learning objectives: To provide an understanding/knowledge of how read and fill a development safety update report after the first Phase I clinical trials.
Key concepts: Rational for writing DSURs; ICH E2F and CIOMS V; Assessment process; DSUR outcomes; Compliance; Benefit/risk balance assessment concept.
Case study(ies)
Speaker: Henri Caplain, Clinical Pharmacologist, Senior Adviser in Early Clinical Development, Translational Pharmacology, and Drug Safety Risk Management, President AFPT-Le Club Phase 1
16:30 – 16:45
Coffee Break
16:45 – 18:30
Learning objectives: To provide the principles of the risk management plan in early drug development.
Key concepts: Risk concept; Crisis management; Impact of AE on drug development and further trials; Risk management plan and planning; Risk evaluation and mitigation strategy; Safety specifications; Important identified and potential risks, missing information; Risk assessment; Risk minimization activities; Risk communication; Effectiveness of risk minimization; DRMP/DSUR progression during drug development; How to fill a risk management plan prior to the CTA/IND.
Case study(ies)
Speaker: Henri Caplain, Clinical Pharmacology, Senior Adviser in Early Clinical Development, Translational Pharmacology, and Drug Safety Risk Management, President AFPT-Le Club Phase 1
18:30
Adjourn
Day 5: Friday 22 March 2024
Management of medical emergencies in human pharmacology trials and exam
09:00 – 10:30
Work by sub-group on a case study
Facilitator: Henri Caplain, Clinical Pharmacology, Senior Adviser in Early Clinical Development, Translational Pharmacology, and Drug Safety Risk Management, President AFPT-Le Club Phase 1
10:30 – 10:45
Coffee Break
10:45 – 12:00
End and reporting
Facilitator: Henri Caplain, Clinical Pharmacology, Senior Adviser in Early Clinical Development, Translational Pharmacology, and Drug Safety Risk Management, President AFPT-Le Club Phase 1
12:00 – 12:30
- Facilitator: Henri Caplain, Clinical Pharmacology, Senior Adviser in Early Clinical Development, Translational Pharmacology, and Drug Safety Risk Management, President AFPT-Le Club Phase 1
12:30 – 14:00
Lunch
14:00 – 16:00
- Selection of multiple-choice questions (1 hour): 60 % of questions must be correctly answered to pass test and receive a certificate.
- Short questions (4 of 15 minutes each): 10/20 must be obtained to pass test and receive a certificate
16:00
End of the training
Attendance Fees
1,600 € + VAT
Members of the AFPT-Le Club Phase 1 and other EUFEMED members (AGAH, AHPPI, HEALIXIA, POLFEMED)
2,100 € + VAT
Non-Members
Payments of a registration fee covers the cost to attend all courses, educational material, coffee breaks, and all lunches during the course. Notice that this registration fee does not cover transportation fee and accommodation fee.
Special fees and free invitation (limited numbers) for students are available on request.
Subject to change without notice.
Contact and further information
Organiser and responsible for the programme
Association Française de Pharmacologie Translationnelle – Le Club Phase 1
34, avenue des Champs-Elysées
75008 Paris
Registration and billing contact
CSi Hamburg GmbH
Goernestraße 30
20249 Hamburg (Germany)